Clinical Services / Clinical Information
Clinical Information
Gateway’s Clinical Services team provides every client with Bulletin Reports, Weekly Exclusives, and monthly Pipeline Reports. Below are examples of the reports we provide.
Bulletin Reports
Example
Cimerli Interchangeable Biosimilar for Luncentis
The U.S. Food and Drug Administration (FDA) approved Coherus BioSciences’ Cimerli™ (ranibizumab-eqrn) injection on Aug. 2, 2022. As the first interchangeable biosimilar for Lucentis® (ranibizumab) to be approved in the United States, it will have one year of exclusivity from its launch date before another interchangeable Lucentis biosimilar can enter the market. Both products are indicated for intravitreal (into the eye) injections for treating neovascular (wet) age-related macular degeneration (nAMD), macular edema after retinal vein occlusion (RVO), diabetic macular edema (DME), diabetic retinopathy (DR) and myopic choroidal neovascularization (mCNV). In general, treatments for the conditions are administered once a month, but dosing intervals can be longer for some patients. Because they are injected into the eyes by specially trained healthcare providers in a sterile medical facility, coverage often falls under medical benefits. Specialty pharmacies also dispense ophthalmic VEGF inhibitors, however. Its release is scheduled to start early in October, but pricing and distribution channels have not yet been announced. Prescribing information for Cimerli is here.
Weekly Exclusive Newsletter
Example
In this weeks newsletter
• Bortezomib Injection
• Kyzatrex® (testosterone undecanoate) capsules
• Zoryve™ (roflumilast) cream
• Regeneron Ends REGEN-COV Studies
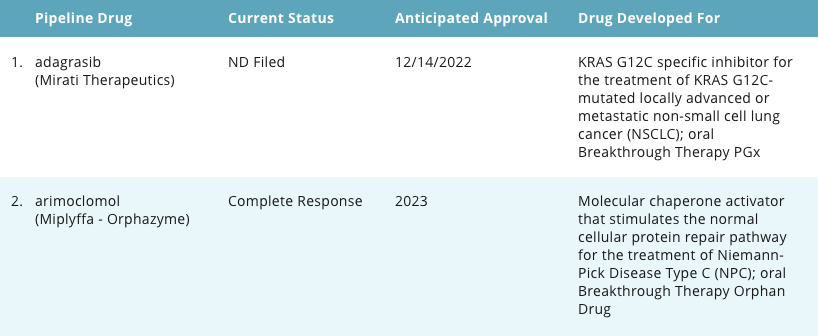
Pipeline Report
Example
Top 20 Specialty Report
Updated May 2022